Introduction
Large granular lymphocytic leukemia (LGLL) is a rare chronic lymphoproliferative disease of T cell and natural killer (NK) cell lineage. Less than 10% of cases are NK-large granular lymphocytic leukemia (NK-LGLL), which is distinguished by clonal growth of mature NK cells that are CD3-, CD16+, and/or CD56+. There are two types of mature NK cells based on CD56 intensity. The CD56dim NK-cell subset exhibits a higher level of natural cytotoxic activity compared to the CD56bright NK-cell subset, which, in turn, is capable of producing abundant cytokines ( Trends Immunol. 2001;22(11):633-640). However, few studies have explored the internal heterogeneity of NK-LGLL based on CD56 expression ( Leukemia. 2010;24(4):881-884; Blood Cancer J. 2018;8(6):51) .
Method
A total of 402 patients have been diagnosed with LGLL at our hospital since June 2005, including 40 with NK-LGLL. All patients met the recommended diagnostic criteria ( Blood. 2017;129(9):1082-1094). Treatment responses were collected after at least 3 months of therapy. NK subtype was defined by flow cytometry analysis using common surface markers showing CD3−/CD16+, or CD56+ pattern, and cells were considered antigen partial positive if antibody staining was between 20%-80%. To perform subgroup analysis, we incorporated partial CD56 positive into the negative group since mature NK cells are CD56-positive. Clonality was evaluated by killer-cell immunoglobulin-like receptors (KIR). Sequencing of STAT3 and STAT5b was performed with Next Generation Sequencing.
Results
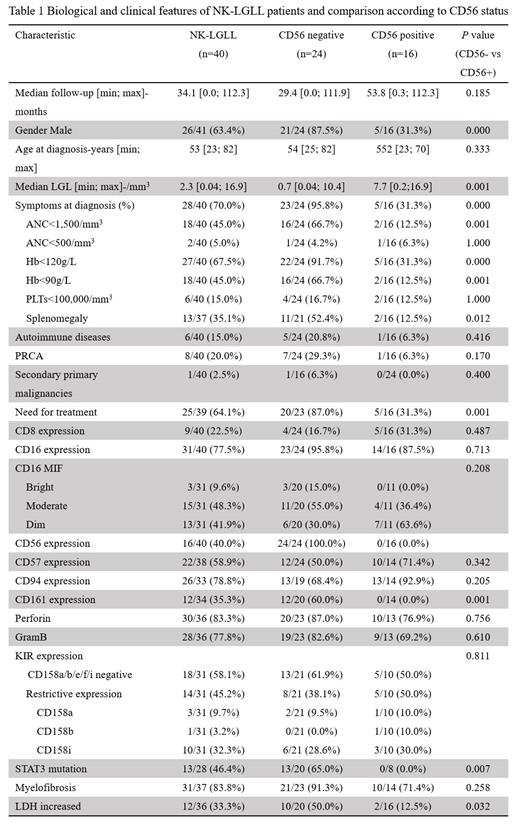
Twenty-eight of 40 patients (70.0%) presented symptoms at the time of diagnosis in which anemia was the most prevalent accounting for 67.5%. PRCA was the most common comorbidity, affecting 20.0% of patients. All cases were CD2+, CD3-. Expressions of mature NK cell markers are heterogenous, including CD8 (22.5%), CD16 (77.5%), CD56 (40.0%), CD57 (58.9%), CD94 (78.8%), CD161 (35.3%), perforin (83.3%), GramB (77.8%). KIR analyses were available in 31 cases, 45.2% of NK-LGLs showed a form of restrictive expression, mainly CD158i (32.3%), an activating KIR, whereas 58.1% of cases lacked CD158a/b/e/f/i expression. STAT3 mutated status was studied in 28 cases (70.0%), 46.4% had STAT3 mutations, and no STAT5B mutations were detected.
We classified 40 cases into two subsets based on CD56 expression (Table 1). CD56- cases displayed a more aggressive course. They usually presented with symptomatic disease including anemia (91.7% vs 31.3%, P= 0.000), severe anemia (66.7% vs 12.5%, P= 0.001), neutropenia (66.7% vs 12.5%, P= 0.001), splenomegaly (52.4% vs 12.5%, P= 0.012), and treatment requirement (87.0% vs 31.3%, P= 0.001). CD56-negative cases expressed CD161 at a higher frequency (60.0% vs 0.0%, P=0.001) while the other mature markers were similar. Interestingly, STAT3 mutation was detected in 13 of 20 (65.0%) in CD56- NK-LGLL, and none of 8 CD56+ NK-LGLL had STAT3 mutation (p=0.007). The treatment pattern was similar between CD56+ vs CD56- NK-LGLL. However, the treatment efficacy was lower in CD56- NK-LGLL, with overall response rates (ORR) of 100% vs 79% and CR (complete response) rate of 80% vs 46%, in CD56+ and CD56- NK-LGLL patients respectively.
Conclusions
NK-LGLL is a heterogenous entity and can be divided into two groups further based on CD56 expression. CD56- NK-LGLL showed more prominent clinical symptoms, high STAT3 mutation, and CD161 expression. CD56- NK-LGLL showed a poor response than CD56+ NK-LGLL. More investigations are deserved to explore the underlying mechanisms.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal